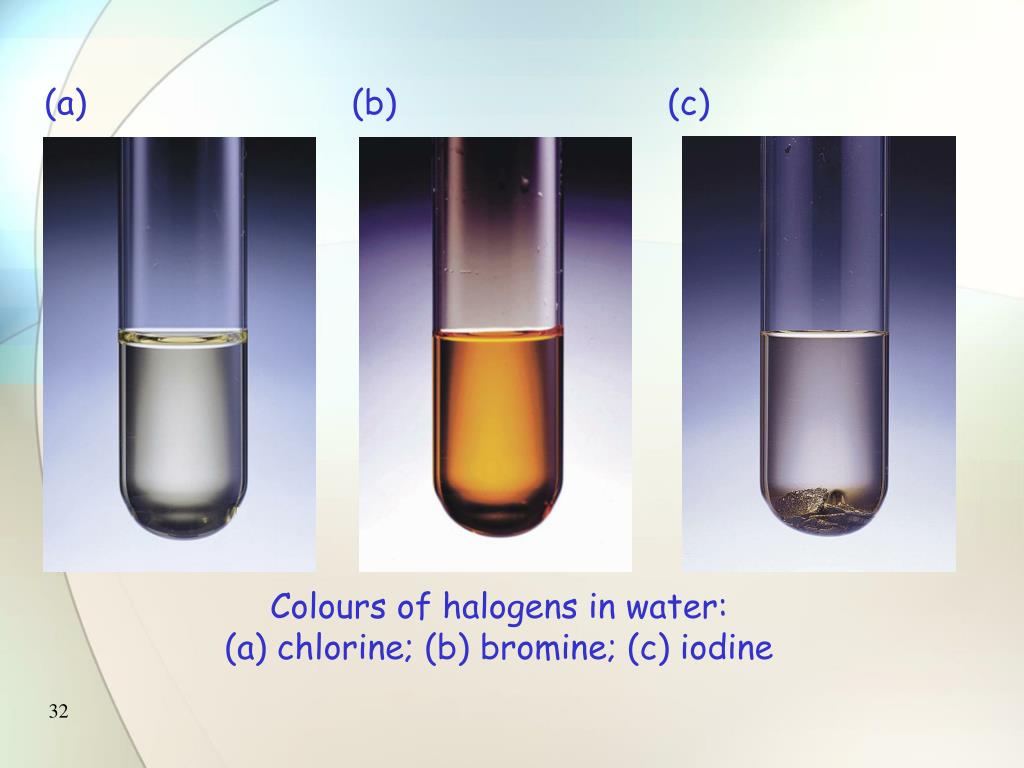
Bromine Hexane Color . A dilute solution of bromine is added to samples of heptane and cyclohexene. for chlorine and bromine the colour does not change. However, for iodine there is a colour. Bromine is a corrosive and poisonous brown liquid. what will be the color of the hexane layer (based on the halogen produced)? the bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Since the product side contains iodine, the hexane. Bromine reacted with hexane to form a colorless. this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between. You might need a white background to see the colour of the chlorine solution. The bromination reactions and mechanisms. When bromine is added to the sample, if the reddish color disappear, that. hexane is a colorless liquid. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the.
from www.slideserve.com
the bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Since the product side contains iodine, the hexane. You might need a white background to see the colour of the chlorine solution. A dilute solution of bromine is added to samples of heptane and cyclohexene. Bromine reacted with hexane to form a colorless. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. what will be the color of the hexane layer (based on the halogen produced)? hexane is a colorless liquid. for chlorine and bromine the colour does not change. Bromine is a corrosive and poisonous brown liquid.
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141
Bromine Hexane Color You might need a white background to see the colour of the chlorine solution. Bromine reacted with hexane to form a colorless. what will be the color of the hexane layer (based on the halogen produced)? if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. for chlorine and bromine the colour does not change. Since the product side contains iodine, the hexane. hexane is a colorless liquid. You might need a white background to see the colour of the chlorine solution. A dilute solution of bromine is added to samples of heptane and cyclohexene. The bromination reactions and mechanisms. this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between. However, for iodine there is a colour. the bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Bromine is a corrosive and poisonous brown liquid. When bromine is added to the sample, if the reddish color disappear, that.
From www.youtube.com
LIGHT INDUCED RADICAL SUBSTITUTION OF BROMINE ON HEXANE YouTube Bromine Hexane Color for chlorine and bromine the colour does not change. However, for iodine there is a colour. When bromine is added to the sample, if the reddish color disappear, that. The bromination reactions and mechanisms. Bromine is a corrosive and poisonous brown liquid. this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions. Bromine Hexane Color.
From www.chemedx.org
Creating a Time Lapse Video for the Bromination of Hexane in the Presence of UV Light Chemical Bromine Hexane Color Since the product side contains iodine, the hexane. hexane is a colorless liquid. When bromine is added to the sample, if the reddish color disappear, that. However, for iodine there is a colour. The bromination reactions and mechanisms. You might need a white background to see the colour of the chlorine solution. the bromine reagent is in reddish. Bromine Hexane Color.
From www.numerade.com
SOLVED Using condensed structure, write the balanced chemical equation for bromination reaction Bromine Hexane Color this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. However, for iodine there is a colour. A dilute solution of bromine is added to samples of heptane and. Bromine Hexane Color.
From www.numerade.com
SOLVED Reaction with Bromine Describe the test results for the Bromine test Color Change HBr Bromine Hexane Color A dilute solution of bromine is added to samples of heptane and cyclohexene. what will be the color of the hexane layer (based on the halogen produced)? You might need a white background to see the colour of the chlorine solution. for chlorine and bromine the colour does not change. Bromine reacted with hexane to form a colorless.. Bromine Hexane Color.
From www.numerade.com
SOLVED Using condensed structure, write the balanced chemical equation for the bromination Bromine Hexane Color However, for iodine there is a colour. Since the product side contains iodine, the hexane. The bromination reactions and mechanisms. Bromine is a corrosive and poisonous brown liquid. Bromine reacted with hexane to form a colorless. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. . Bromine Hexane Color.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway C41 ab Halogen physical properties Bromine Hexane Color what will be the color of the hexane layer (based on the halogen produced)? Bromine reacted with hexane to form a colorless. A dilute solution of bromine is added to samples of heptane and cyclohexene. Since the product side contains iodine, the hexane. hexane is a colorless liquid. You might need a white background to see the colour. Bromine Hexane Color.
From www.numerade.com
SOLVED What changes in color occur when bromine reacts with an alkene? What changes in color Bromine Hexane Color if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. hexane is a colorless liquid. You might need a white background to see the colour of the chlorine solution. for chlorine and bromine the colour does not change. Bromine reacted with hexane to form a. Bromine Hexane Color.
From www.numerade.com
SOLVED EXPERIMENT Data and Report Sheet HYDROCARBONS A.Reaction with Bromine Table 1 (data Bromine Hexane Color what will be the color of the hexane layer (based on the halogen produced)? this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between. the bromine reagent is in reddish color, and the product vicinal dibromide is colorless. for chlorine and bromine the colour does not change. Since the. Bromine Hexane Color.
From www.slideserve.com
PPT Group 18 the noble gases PowerPoint Presentation ID6382540 Bromine Hexane Color this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between. However, for iodine there is a colour. hexane is a colorless liquid. You might need a white background to see the colour of the chlorine solution. for chlorine and bromine the colour does not change. the bromine reagent is. Bromine Hexane Color.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Bromine Hexane Color the bromine reagent is in reddish color, and the product vicinal dibromide is colorless. A dilute solution of bromine is added to samples of heptane and cyclohexene. The bromination reactions and mechanisms. Bromine is a corrosive and poisonous brown liquid. You might need a white background to see the colour of the chlorine solution. this page gives you. Bromine Hexane Color.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5566024 Bromine Hexane Color Since the product side contains iodine, the hexane. what will be the color of the hexane layer (based on the halogen produced)? You might need a white background to see the colour of the chlorine solution. Bromine is a corrosive and poisonous brown liquid. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will. Bromine Hexane Color.
From quintensrtate.blogspot.com
Colour of Bromine Water QuintensrTate Bromine Hexane Color what will be the color of the hexane layer (based on the halogen produced)? if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. Since the product side contains iodine, the hexane. However, for iodine there is a colour. A dilute solution of bromine is added. Bromine Hexane Color.
From pixels.com
Alkene Bromine Test Photograph by Andrew Lambert Photography Pixels Bromine Hexane Color When bromine is added to the sample, if the reddish color disappear, that. You might need a white background to see the colour of the chlorine solution. the bromine reagent is in reddish color, and the product vicinal dibromide is colorless. what will be the color of the hexane layer (based on the halogen produced)? A dilute solution. Bromine Hexane Color.
From www.youtube.com
Bromination of Hexane in the Presence of UV Light YouTube Bromine Hexane Color this page gives you the facts and a simple, uncluttered mechanism for the electrophilic addition reactions between. The bromination reactions and mechanisms. However, for iodine there is a colour. Bromine is a corrosive and poisonous brown liquid. what will be the color of the hexane layer (based on the halogen produced)? You might need a white background to. Bromine Hexane Color.
From ceffqzmr.blob.core.windows.net
Bromine Color Change at Kim Nielsen blog Bromine Hexane Color Bromine is a corrosive and poisonous brown liquid. A dilute solution of bromine is added to samples of heptane and cyclohexene. Since the product side contains iodine, the hexane. The bromination reactions and mechanisms. You might need a white background to see the colour of the chlorine solution. for chlorine and bromine the colour does not change. hexane. Bromine Hexane Color.
From www.numerade.com
SOLVED If you used a bromine test to distinguish between hexane and 2hexene, in which would th Bromine Hexane Color what will be the color of the hexane layer (based on the halogen produced)? Since the product side contains iodine, the hexane. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. for chlorine and bromine the colour does not change. Bromine reacted with hexane. Bromine Hexane Color.
From www.numerade.com
SOLVED Using condensed structure, write the balanced chemical equation for the bromination Bromine Hexane Color what will be the color of the hexane layer (based on the halogen produced)? for chlorine and bromine the colour does not change. However, for iodine there is a colour. if the hydrocarbon is hexane, no reaction will occur and the hydrocarbon solution will immediately take on the brown color of the. You might need a white. Bromine Hexane Color.
From www.joelgordon.com
Bromine + Hydrocarbon Joel Gordon Photography Bromine Hexane Color The bromination reactions and mechanisms. hexane is a colorless liquid. Since the product side contains iodine, the hexane. You might need a white background to see the colour of the chlorine solution. A dilute solution of bromine is added to samples of heptane and cyclohexene. However, for iodine there is a colour. if the hydrocarbon is hexane, no. Bromine Hexane Color.